About
Aims
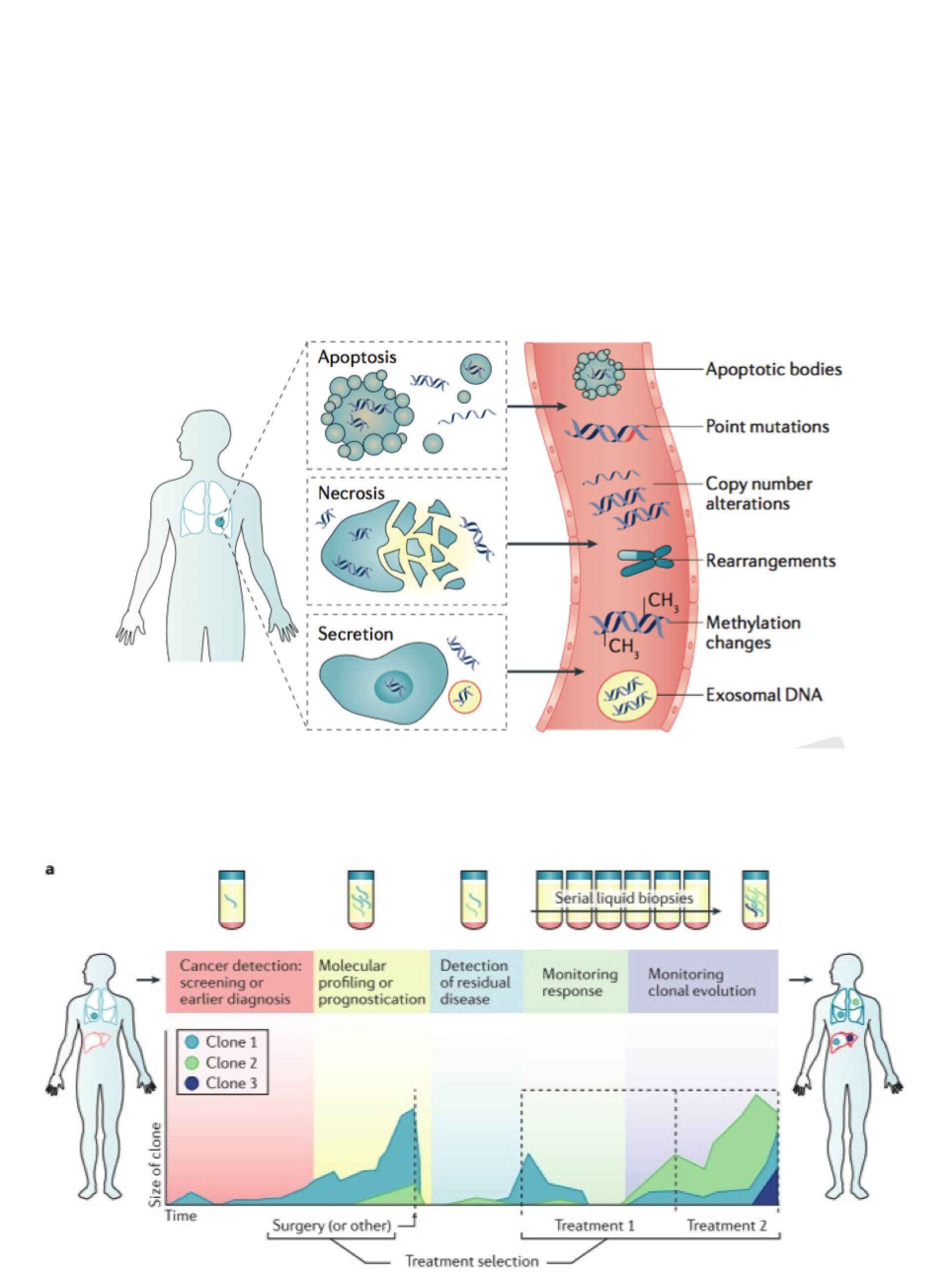
The Rosenfeld Lab employs emerging molecular technologies to develop new diagnostic approaches. Our focus is on circulating tumour DNA (ctDNA) as a noninvasive modality to assess evolution of solid malignancies. This is DNA originating from cancer cells, carrying tumour-specific genomic alterations, that is present as short cell-free fragments in body fluids such as blood plasma. ctDNA can be collected noninvasively via blood samples and has the potential to be immensely informative.
Recent Publications
Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, Rundell V, Wulff J, Sharma G, Knock H, Castedo J, Cooper W, Zhao H, Smith CG, Garg S, Anand S, Howarth K, Gilligan D, Harden SV, Rassl DM, Rintoul RC, Rosenfeld N. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. 2022 May;33(5):500-510. DOI: 10.1016/j.annonc.2022.02.007
Markus H, Chandrananda D, Moore E, Mouliere F, Morris J, Brenton JD, Smith CG, Rosenfeld N. Refined characterization of circulating tumor DNA through biological feature integration. Sci Rep. 2022 Feb 4;12(1):1928. DOI: 10.1038/s41598-022-05606-z
Hudecova I, Smith CG, Hänsel-Hertsch R, Chilamakuri CS, Morris JA, Vijayaraghavan A, Heider K, Chandrananda D, Cooper WN, Gale D, Garcia-Corbacho J, Pacey S, Baird RD, Rosenfeld N, Mouliere F. Characteristics, origin, and potential for cancer diagnostics of ultrashort plasma cell-free DNA. Genome Res. 2022 Feb;32(2):215-227. DOI: 10.1101/gr.275691.121
See all publications
The Rosenfeld Lab employs emerging molecular technologies to develop new diagnostic approaches. Our focus is on circulating tumour DNA (ctDNA) as a noninvasive modality to assess evolution of solid malignancies. This is DNA originating from cancer cells, carrying tumour-specific genomic alterations, that is present as short cell-free fragments in body fluids such as blood plasma. ctDNA can be collected noninvasively via blood samples and has the potential to be immensely informative.
Recent Publications
Gale D, Heider K, Ruiz-Valdepenas A, Hackinger S, Perry M, Marsico G, Rundell V, Wulff J, Sharma G, Knock H, Castedo J, Cooper W, Zhao H, Smith CG, Garg S, Anand S, Howarth K, Gilligan D, Harden SV, Rassl DM, Rintoul RC, Rosenfeld N. Residual ctDNA after treatment predicts early relapse in patients with early-stage non-small cell lung cancer. Ann Oncol. 2022 May;33(5):500-510. DOI: 10.1016/j.annonc.2022.02.007
Markus H, Chandrananda D, Moore E, Mouliere F, Morris J, Brenton JD, Smith CG, Rosenfeld N. Refined characterization of circulating tumor DNA through biological feature integration. Sci Rep. 2022 Feb 4;12(1):1928. DOI: 10.1038/s41598-022-05606-z
Hudecova I, Smith CG, Hänsel-Hertsch R, Chilamakuri CS, Morris JA, Vijayaraghavan A, Heider K, Chandrananda D, Cooper WN, Gale D, Garcia-Corbacho J, Pacey S, Baird RD, Rosenfeld N, Mouliere F. Characteristics, origin, and potential for cancer diagnostics of ultrashort plasma cell-free DNA. Genome Res. 2022 Feb;32(2):215-227. DOI: 10.1101/gr.275691.121
See all publications